Explain the types of distillation pdf Swan Island (Victoria)
Distillation Distillation Vapor scribd.com Fractional distillation is the process of separating a mixture into its different components. It’s similar to simple distillation in that it uses heat (evaporation) and cooling (condensation) to separate substances; the difference is that simple distillation does this process once, while fractional distillation repeats the process several
Distillation Definition in Chemistry ThoughtCo
APPENDIX 1 Food and Agriculture Organization. While reverse osmosis may be used for all types of salt water, the nano-filtration and electro-dialysis techniques are more suitable for brackish water. 2.2. Evaporative Techniques Traditionally, evaporation techniques, especially MSF, have controlled the market of desalination techniques. Since 2004,, this trend has changed since reverse osmosis has proven to work properly and consume less, ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire..
D 1 This is how the distillation process in the fractionator works. E Most of the fractions in the crude oil evaporate. F The condensed liquid flows out of the fractionator through a pipe from the tray. G High-pressure steam is used to heat the crude oil to a high temperature. H The crude oil vapour enters the fractionator and rises up the column. 4 Complete these sentences using each word What happens, and this is the crux of the distillation process, is that the mixture boils at some temperature depending on the relative concentrations and produces a vapour that is a …
The distillation process mimics the natural water cycle in that salt water is heated, producing water vapor that is in turn condensed to form fresh water. In a laboratory or Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of
Structured packing The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns. The result is a very open honeycomb structure with inclined flow channels giving a relatively high surface area but with very low resistance to gas flow. D 1 This is how the distillation process in the fractionator works. E Most of the fractions in the crude oil evaporate. F The condensed liquid flows out of the fractionator through a pipe from the tray. G High-pressure steam is used to heat the crude oil to a high temperature. H The crude oil vapour enters the fractionator and rises up the column. 4 Complete these sentences using each word
Distillation definition, the volatilization or evaporation and subsequent condensation of a liquid, as when water is boiled in a retort and the steam is condensed in a cool receiver. See more. APPENDIX 1 BASIC PRINCIPLES OF STEAM DISTILLATION. Most essential oils are obtained from the plant material by a process known as steam distillation.
Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from Distillation design and operation need to be based on a clear understanding of these phenomena, “energy efficiency” and the process economics of distillation processes and the associated energy-supply processes.
Fractional distillation is the process of separating a mixture into its different components. It’s similar to simple distillation in that it uses heat (evaporation) and cooling (condensation) to separate substances; the difference is that simple distillation does this process once, while fractional distillation repeats the process several The distillation process mimics the natural water cycle in that salt water is heated, producing water vapor that is in turn condensed to form fresh water. In a laboratory or
12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances. Vacuum distillation is used with or without heating the solution.Vacuum Distillation Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure (usually less than atmospheric pressure) causing evaporation of the most volatile liquid(s) (those with the lowest boiling points). Vacuum distillation is
Compared to other separation methods, such as fractional distillation, simple distillation uses less energy. This is because it uses simple apparatus which consists of only a distilling pot and a condenser, an adapter and a receiver. here is based on an ASTM D86 distillation with a 5/95 gap because it uses the 5 volume percent point of the heavier product and the 95 volume percent point of the lighter product. This definition is the one most commonly used in the industry. However, various plants use alternate percent points, weight percent instead of volume percent, or use different ASTM distillation methods. All these
Distillation, sometimes referred to as fractionation or rectification, is a process for the separating of two or more liquids. The process utilizes the difference of the 12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances.
Steam distillation is a much gentler method of achieving the same end. In steam distillation, the distilling pot is infused with steam, which carries the Oil’s vapor into the distilling head and then into the condenser, where the Oil and Water co-condense. As Compared to other separation methods, such as fractional distillation, simple distillation uses less energy. This is because it uses simple apparatus which consists of only a distilling pot and a condenser, an adapter and a receiver.
Distillation Distillation Vapor scribd.com

Distillation Distillation Vapor scribd.com. Distillation is commonly done in one of two ways, on a continuous basis in a large, industrial scale still, or on a batch basis, in a small artisanal still., The review of theory presented below is based on Ref. [1]. Column at Total Reflux The first experiment with both the batch and continuous distillation systems should be performed at total reflux in order to obtain the column efficiency. The overall column efficiency is defined as рќ‘’рќ‘џрќ‘Ћ Гџ Гџ= рќ‘Ѓ в„Ћрќ‘’рќ‘џрќ‘’ рќ‘–рќ‘ђрќ‘Ћ Гџ рќ‘Ѓрќ‘Ћрќ‘ђ рќ‘Ћ Гџ (3) where N actual is the actual.
Distillation Define Distillation at Dictionary.com. Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from, Structured packing The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns. The result is a very open honeycomb structure with inclined flow channels giving a relatively high surface area but with very low resistance to gas flow..
Distillation Definition in Chemistry ThoughtCo

Types of Distillation Columns Consulting Engineers. Extractive Distillation Extractive distillation works because an heavy solvent is specially chosen to interact differently with the components of the original mixture, thereby altering The Methods of Extracting Essential Oils include expression, steam distillation, solvent extraction and super critical fluid extraction. Find out what is the best for producing therpeutic grade oils!.

Structured packing The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns. The result is a very open honeycomb structure with inclined flow channels giving a relatively high surface area but with very low resistance to gas flow. ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
Distillation, sometimes referred to as fractionation or rectification, is a process for the separating of two or more liquids. The process utilizes the difference of the APPENDIX 1 BASIC PRINCIPLES OF STEAM DISTILLATION. Most essential oils are obtained from the plant material by a process known as steam distillation.
The review of theory presented below is based on Ref. [1]. Column at Total Reflux The first experiment with both the batch and continuous distillation systems should be performed at total reflux in order to obtain the column efficiency. The overall column efficiency is defined as рќ‘’рќ‘џрќ‘Ћ Гџ Гџ= рќ‘Ѓ в„Ћрќ‘’рќ‘џрќ‘’ рќ‘–рќ‘ђрќ‘Ћ Гџ рќ‘Ѓрќ‘Ћрќ‘ђ рќ‘Ћ Гџ (3) where N actual is the actual Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from
Distillation is commonly done in one of two ways, on a continuous basis in a large, industrial scale still, or on a batch basis, in a small artisanal still. ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
While reverse osmosis may be used for all types of salt water, the nano-filtration and electro-dialysis techniques are more suitable for brackish water. 2.2. Evaporative Techniques Traditionally, evaporation techniques, especially MSF, have controlled the market of desalination techniques. Since 2004,, this trend has changed since reverse osmosis has proven to work properly and consume less APPENDIX 1 BASIC PRINCIPLES OF STEAM DISTILLATION. Most essential oils are obtained from the plant material by a process known as steam distillation.
Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from Compared to other separation methods, such as fractional distillation, simple distillation uses less energy. This is because it uses simple apparatus which consists of only a distilling pot and a condenser, an adapter and a receiver.
While reverse osmosis may be used for all types of salt water, the nano-filtration and electro-dialysis techniques are more suitable for brackish water. 2.2. Evaporative Techniques Traditionally, evaporation techniques, especially MSF, have controlled the market of desalination techniques. Since 2004,, this trend has changed since reverse osmosis has proven to work properly and consume less ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
Distillation design and operation need to be based on a clear understanding of these phenomena, “energy efficiency” and the process economics of distillation processes and the associated energy-supply processes. The review of theory presented below is based on Ref. [1]. Column at Total Reflux The first experiment with both the batch and continuous distillation systems should be performed at total reflux in order to obtain the column efficiency. The overall column efficiency is defined as 𝑒𝑟𝑎 ß ß= 𝑁 ℎ𝑒𝑟𝑒 𝑖𝑐𝑎 ß 𝑁𝑎𝑐 𝑎 ß (3) where N actual is the actual
Structured packing The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns. The result is a very open honeycomb structure with inclined flow channels giving a relatively high surface area but with very low resistance to gas flow. What happens, and this is the crux of the distillation process, is that the mixture boils at some temperature depending on the relative concentrations and produces a vapour that is a …
Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances. Explain the principles and apparatus used in simple distillation Analyse the concentration & temperature changes with time Derive Rayleigh Equation for simple distillation
Distillation Guide Organic Chemistry Help!
APPENDIX 1 Food and Agriculture Organization. 12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances., While reverse osmosis may be used for all types of salt water, the nano-filtration and electro-dialysis techniques are more suitable for brackish water. 2.2. Evaporative Techniques Traditionally, evaporation techniques, especially MSF, have controlled the market of desalination techniques. Since 2004,, this trend has changed since reverse osmosis has proven to work properly and consume less.
Distillation Definition in Chemistry ThoughtCo
Distillation Distillation Vapor scribd.com. The review of theory presented below is based on Ref. [1]. Column at Total Reflux The first experiment with both the batch and continuous distillation systems should be performed at total reflux in order to obtain the column efficiency. The overall column efficiency is defined as рќ‘’рќ‘џрќ‘Ћ Гџ Гџ= рќ‘Ѓ в„Ћрќ‘’рќ‘џрќ‘’ рќ‘–рќ‘ђрќ‘Ћ Гџ рќ‘Ѓрќ‘Ћрќ‘ђ рќ‘Ћ Гџ (3) where N actual is the actual, Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from.
Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from Simple Distillation - In simple distillation, vapor enters a condenser, cools, and is collected. The resulting liquid has a composition identical to that of the vapor, so simple distillation is used when components have greatly different boiling points or to separate volatile from non-volatile components.
What happens, and this is the crux of the distillation process, is that the mixture boils at some temperature depending on the relative concentrations and produces a vapour that is a … here is based on an ASTM D86 distillation with a 5/95 gap because it uses the 5 volume percent point of the heavier product and the 95 volume percent point of the lighter product. This definition is the one most commonly used in the industry. However, various plants use alternate percent points, weight percent instead of volume percent, or use different ASTM distillation methods. All these
D 1 This is how the distillation process in the fractionator works. E Most of the fractions in the crude oil evaporate. F The condensed liquid flows out of the fractionator through a pipe from the tray. G High-pressure steam is used to heat the crude oil to a high temperature. H The crude oil vapour enters the fractionator and rises up the column. 4 Complete these sentences using each word Binous - Introd. to Distillation. 33. Distillation Equipment. Sieve tray, is the most common arrangement used. It is cheap, simple, and well understood in terms of its performance. Valve arrangement offer more flexibility to cope with wider rangeof liquid and vaporflow rates. Binous - Introd. to Distillation 34. Binous - Introd. to Distillation 35. The difference between the performance of an
Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
Explain the principles and apparatus used in simple distillation Analyse the concentration & temperature changes with time Derive Rayleigh Equation for simple distillation The distillation process mimics the natural water cycle in that salt water is heated, producing water vapor that is in turn condensed to form fresh water. In a laboratory or
Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of
12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances. Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from
A schematic of a typical distillation unit with a single feed and two product streams is shown below: Basic Operation and Terminology The liquid mixture that is to be processed is known as the feed and this is introduced usually somewhere near the middle of the column to a tray known as the feed tray . Simple Distillation - In simple distillation, vapor enters a condenser, cools, and is collected. The resulting liquid has a composition identical to that of the vapor, so simple distillation is used when components have greatly different boiling points or to separate volatile from non-volatile components.
Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of The distillation process mimics the natural water cycle in that salt water is heated, producing water vapor that is in turn condensed to form fresh water. In a laboratory or
Steam distillation is a much gentler method of achieving the same end. In steam distillation, the distilling pot is infused with steam, which carries the Oil’s vapor into the distilling head and then into the condenser, where the Oil and Water co-condense. As The Methods of Extracting Essential Oils include expression, steam distillation, solvent extraction and super critical fluid extraction. Find out what is the best for producing therpeutic grade oils!
Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from
Distillation Distillation Vapor scribd.com
The Advantages of Simple Distillation Sciencing. Fractional distillation is the process of separating a mixture into its different components. It’s similar to simple distillation in that it uses heat (evaporation) and cooling (condensation) to separate substances; the difference is that simple distillation does this process once, while fractional distillation repeats the process several, Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from.
Distillation Guide Organic Chemistry Help!. Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of, Distillation, sometimes referred to as fractionation or rectification, is a process for the separating of two or more liquids. The process utilizes the difference of the.
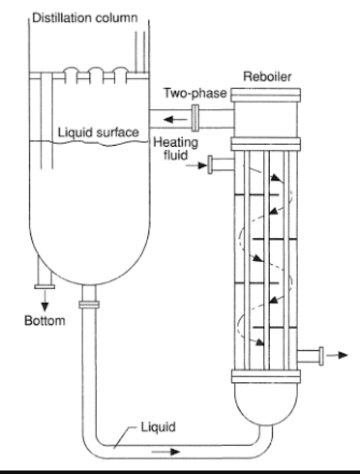
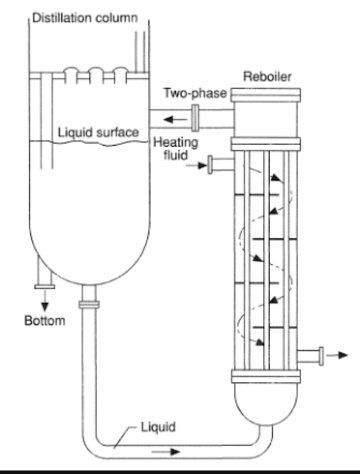
Distillation Column Column Internals Bubble cap trays

Desalination Technologies (I) CRES. While reverse osmosis may be used for all types of salt water, the nano-filtration and electro-dialysis techniques are more suitable for brackish water. 2.2. Evaporative Techniques Traditionally, evaporation techniques, especially MSF, have controlled the market of desalination techniques. Since 2004,, this trend has changed since reverse osmosis has proven to work properly and consume less Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from.

here is based on an ASTM D86 distillation with a 5/95 gap because it uses the 5 volume percent point of the heavier product and the 95 volume percent point of the lighter product. This definition is the one most commonly used in the industry. However, various plants use alternate percent points, weight percent instead of volume percent, or use different ASTM distillation methods. All these A schematic of a typical distillation unit with a single feed and two product streams is shown below: Basic Operation and Terminology The liquid mixture that is to be processed is known as the feed and this is introduced usually somewhere near the middle of the column to a tray known as the feed tray .
The Methods of Extracting Essential Oils include expression, steam distillation, solvent extraction and super critical fluid extraction. Find out what is the best for producing therpeutic grade oils! The Methods of Extracting Essential Oils include expression, steam distillation, solvent extraction and super critical fluid extraction. Find out what is the best for producing therpeutic grade oils!
Binous - Introd. to Distillation. 33. Distillation Equipment. Sieve tray, is the most common arrangement used. It is cheap, simple, and well understood in terms of its performance. Valve arrangement offer more flexibility to cope with wider rangeof liquid and vaporflow rates. Binous - Introd. to Distillation 34. Binous - Introd. to Distillation 35. The difference between the performance of an here is based on an ASTM D86 distillation with a 5/95 gap because it uses the 5 volume percent point of the heavier product and the 95 volume percent point of the lighter product. This definition is the one most commonly used in the industry. However, various plants use alternate percent points, weight percent instead of volume percent, or use different ASTM distillation methods. All these
Distillation under reduced pressure Distillation under reduced pressure is a process in which the liquid is distilled at a temp lower than its boilng point by the application of vacuum. Liquid boils – Vapour pressure = atmospheric pressure , if atmosphric pressure reduced by applying vaccum the bp of liquid decreases. Thefore liquid boils at lower temp. Mass of vapour formed α vp of The Methods of Extracting Essential Oils include expression, steam distillation, solvent extraction and super critical fluid extraction. Find out what is the best for producing therpeutic grade oils!
12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances. Distillation is a method of separating the constituents of mixture (either a liquid or q gaseous one). It is a physical process and not a chemical reaction using the different boiling temperatures of the constituents to separate them from the others. In the rhum agricole context, we are dealing with alcoholic distillation, a way of separation alcohol from water. Alcohol can be segregated from
Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from Fractional distillation is the process of separating a mixture into its different components. It’s similar to simple distillation in that it uses heat (evaporation) and cooling (condensation) to separate substances; the difference is that simple distillation does this process once, while fractional distillation repeats the process several
Steam distillation is a much gentler method of achieving the same end. In steam distillation, the distilling pot is infused with steam, which carries the Oil’s vapor into the distilling head and then into the condenser, where the Oil and Water co-condense. As Distillation is commonly done in one of two ways, on a continuous basis in a large, industrial scale still, or on a batch basis, in a small artisanal still.
Distillation: Distillation, process involving the conversion of a liquid into vapour that is subsequently condensed back to liquid form. It is exemplified at its simplest when steam from a kettle becomes deposited as drops of distilled water on a cold surface. Distillation is used to separate liquids from ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire.
ORGANIC LABORATORY TECHNIQUES 10 10.1 DISTILLATION NEVER distill the distillation flask to dryness as there is a risk of explosion and fire. 12/06/2014В В· Different types of distillation are simple, fractional, steam, vacuum and short path distillation. Distillation is a physical method of assorting mixtures depending upon the difference in the boiling point of the component substances.
APPENDIX 1 BASIC PRINCIPLES OF STEAM DISTILLATION. Most essential oils are obtained from the plant material by a process known as steam distillation. Structured packing The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns. The result is a very open honeycomb structure with inclined flow channels giving a relatively high surface area but with very low resistance to gas flow.
A schematic of a typical distillation unit with a single feed and two product streams is shown below: Basic Operation and Terminology The liquid mixture that is to be processed is known as the feed and this is introduced usually somewhere near the middle of the column to a tray known as the feed tray . Distillation design and operation need to be based on a clear understanding of these phenomena, “energy efficiency” and the process economics of distillation processes and the associated energy-supply processes.


